14+ Chapter 7 Study Guide Ionic Compounds And Metals
Chemical Changes and Energy. ResearchGate is a network dedicated to science and research.
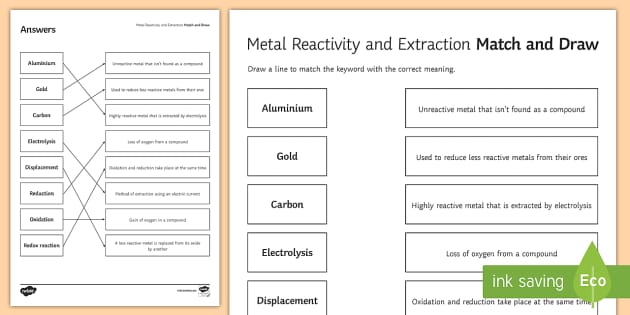
Metal Reactivity And Extraction Match And Draw Twinkl
Download Chapter-wise NCERT Solutions for Class 12 Chemistry.

. Amorphous solids are solids without a regulardefinitive arrangement of its constituent particles ions atoms or molecules and they possess something called the short-range order ie a regular and periodically repeating arrangement is seen only over short distances eg rubber glass. December 10 2022 4. NCERT Exemplar Solutions Class 10 Science Chapter 4 Free PDF Download.
Student Study Guide Chapter 7. Chemical Formula for Ionic Compounds. Studying this NCERT will help you make your foundation strong and you.
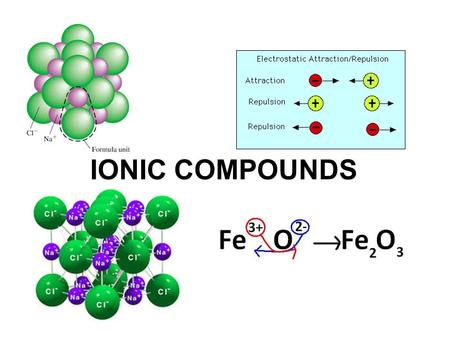
For example sodium chloride melts at 801 C and boils at 1413 C. Chemical Reactions and Chemical Equations. An ionic compound always contains an equal magnitude of positive and negative charges.
So BeSO 4 is more soluble than ionic BaSO 4. Regulators are leaning toward torpedoing the Activision Blizzard deal. Ionic compounds are solids that typically melt at high temperatures and boil at even higher temperatures.
Extracting Metals Low in the Activity Series. H 3 O OH 2H 2 O. To determine when bonds are ionic polar covalent or non-polar covalent these options are known as ionic character it is essential to have a grasp on the.
Define the term amorphous. Study Guide Midterm Exam 2. But the smaller entities like beryllium have higher charge density resulting in higher solvation and hence release of hydration enthalpy larger than the dissociating energy.
It is the first element in group 12 IIB of the periodic tableIn some respects zinc is chemically similar to magnesium. Ionic compounds are the result of a metal chemically bonding with a non-metal. Cool online chemistry videos dictionary tools etc.
December 7 2022 4. Compared to other metals in this category it has an unusually high melting point 2042 K v 1338 for gold. Give a few examples of amorphous solids.
Subtopics of Class 12 Chemistry. The course content outlined below is organized into commonly taught units of study that provide one possible sequence for the course. Zinc is a slightly brittle metal at room temperature and has a shiny-greyish appearance when oxidation is removed.
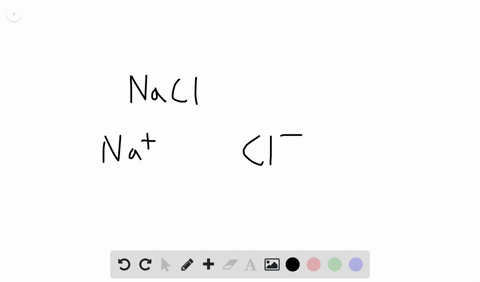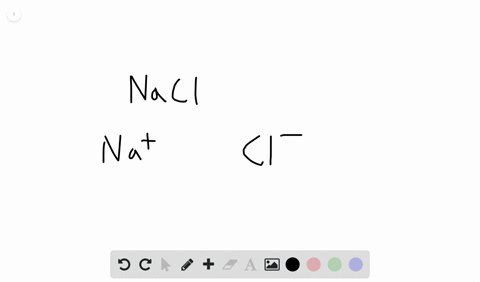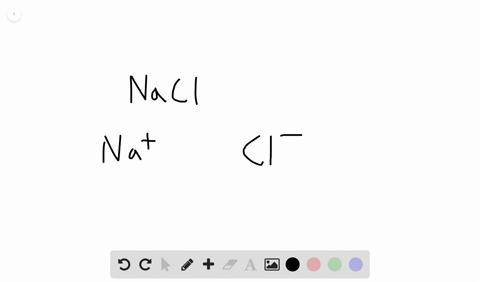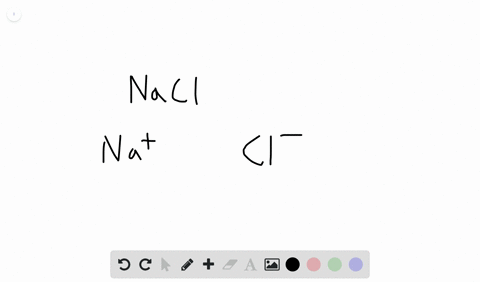
Extracting Metals towards the Top of the Activity. Are usually crystalline. The two parts of the compounds the metal and the non-metal are known as the.
The table salt you might use is an ionic compound and is officially. Hello and welcome to Protocol Entertainment your guide to the business of the gaming and media industries. Properties of Ionic Compounds.
As a comparison the molecular compound water melts at 0 C and boils at 100 C. Checklist for Chapter 7. Vesttoos 80 Million Investment Round.
HowStuffWorks explains thousands of topics from engines to lock-picking to ESP with video and illustrations so you can learn how everything works. Valence electrons and ionic compounds. The hydroxide ion is a natural part of water because of the self-ionization reaction in which its complement hydronium is passed hydrogen.
HOW DO METALS AND NON-METALS REA ALS REACT. The best guide to the covalent or ionic character of a bond is to consider the types of atoms involved and their relative positions in the periodic table. Extracting Metals in the Middle of the Activity Series.
Properties of Ionic Compounds. It is the best reference material when it comes to learning and understanding complex conceptsproblems. Liquid Water and Water Solutions.
Aura Investments Bonds Private Placement. Connect collaborate and discover scientific publications jobs and conferences. C a C l 2 N a C l K 2 S O 4 etc.
Chapter 7 Glossary Quiz. The equilibrium constant for this reaction defined as. Molecular and Ionic Compound Structure and Properties Youll discover the range of chemical bonds and how their structure can affect the properties of the.
Learn more about the definition of a binary compound and find out examples of binary compounds. Chapter 7 p-Block Elements of Class 12 Chemistry is curated as per the CBSE Syllabus for 2022-23. Ionic Character and Electronegativity.
Ionic compounds are abundant in our world. This Friday were taking a look at Microsoft and Sonys increasingly bitter feud over Call of Duty and whether UK. K w H OH.
Asia. Maadaney Gourmets Dispute Against Motion to Certify Class Action. December 5 2022 4.
You can often recognize ionic compounds because of their properties. Award winning periodic table with user-friendly element data and facts. The NCERT Solutions for Class 12 are provided here for better understanding and clarification of the concepts.
IPad Android and Kindle version. Zinc is a chemical element with the symbol Zn and atomic number 30. 7 The labeling required under 80910b of this chapter must include a separate description of the following sensor performance data observed in the clinical study performed in conformance with paragraph b1 of this section for each intended use population in addition to separate sensor performance data for each different iCGM.
Likewise the Na and Cl atoms in NaCl have an electronegativity difference of 21 and the Mn and I atoms in MnI 2 have a difference of 10 yet both of these substances form ionic compounds. Classify the following compounds as ionic or covalent. Ionic compounds are chemical compounds in which oppositely charged ions are held together by electrostatic forces called ionic bonds.
December 5 2022 4. Platinum is more ductile than gold silver or copper thus being the most ductile of pure metals but it is less malleable than gold. NCERT Solutions for Class 12 Chemistry are drafted by the faculty at BYJUS to help students learn all the complex concepts efficientlyEach and every question from the NCERT Textbook is answered in a systematic format to help students learn in a shorter duration.
A binary compound is a chemical compound that is composed of two elements. Ionic compounds are made of a cation and a negatively charged ion known as an anion. Covalent compounds like beryllium sulphate have a higher enthalpy of dissociation than ionic barium sulphate.
Platinum is a moderately hard metal MH 35 of low mechanical strength with a close-packed face-centred cubic structure BCN 12. The alkaline earth metals are found in Group 2 and include Be Mg Ca Sr Ba and Ra. NCERT Exemplar Class 10 Science Chapter 4 Carbon and Its Compounds is an important study material required by students to gain abundant knowledge on the topic covered in CBSE Class 10 Chapter 4 syllabus.
Has a value close to 10 14 at 25 C so the concentration of hydroxide ions in pure water is close to 10 7 moldm. Both elements exhibit only one normal oxidation state 2 and the Zn 2 and.
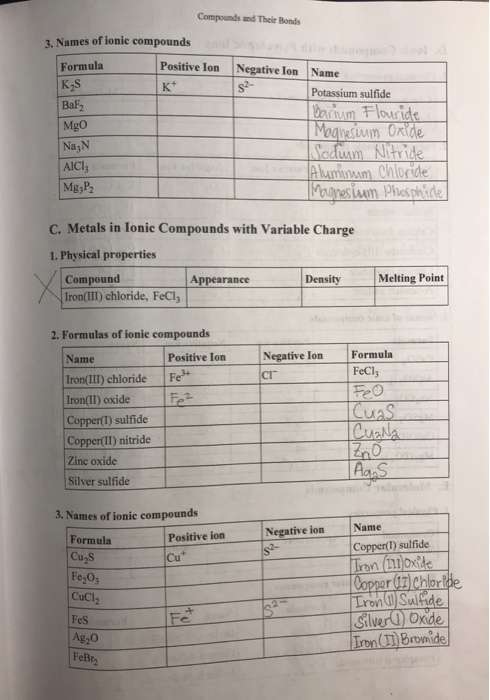
Solved Compounds And Their Bonds 3 Names Of Ionic Compounds Chegg Com

Electrically Conductive Metal Organic Frameworks Chemical Reviews
Ionic Compounds Barbaraelam Rice

Production Of Fuels And Chemicals From Biomass Condensation Reactions And Beyond Chem

Covalent Bonding Worksheet With Answer Key Beyond Science

Chapter 7 Assessment Pdf Ion Ionic Bonding
Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade

Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade

Multitopic Metal Organic Carboxylates Available As Supramolecular Building Units Sciencedirect

7 Study Guide
Chapter 7 Ionic Compounds And Metals Chemistry Matter And Change Ppt Download

Chemistry Mcgraw Hill Chapter 7 Ionic Compounds And Metals Flashcards Quizlet
Pyrimidopyridine Compound Used As A Csbp Rk P38 Modulator Patent 2436686
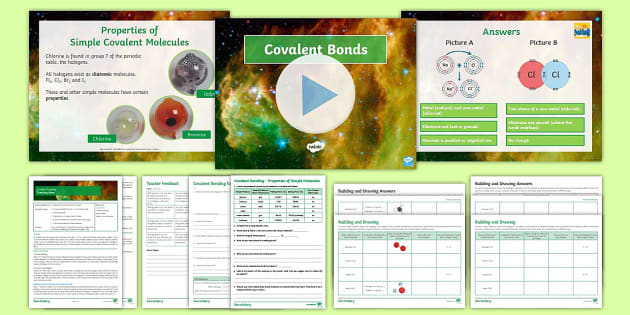
Aqa Bonding Structure And Properties L3 Covalent Bonding
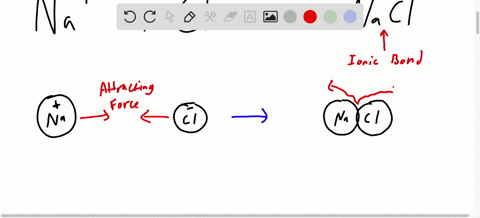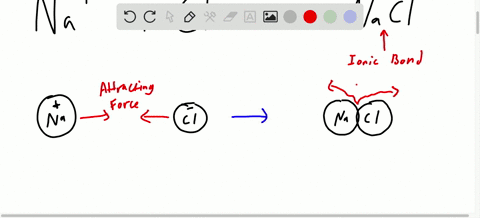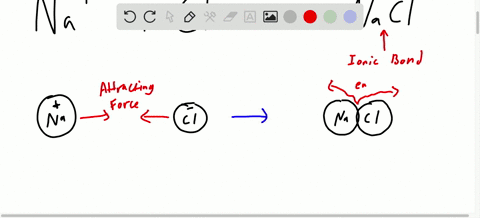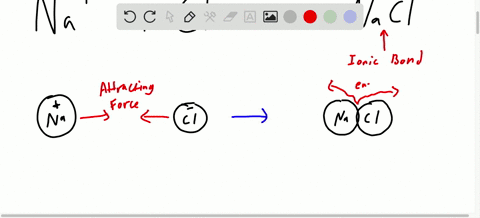
Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade

Chapter 7 Ionic Compounds And Metals Chemistry Matter And Change Ppt Download
Chapter 7 Ionic Compounds And Metals Video Solutions Chemistry Matter And Change Numerade